
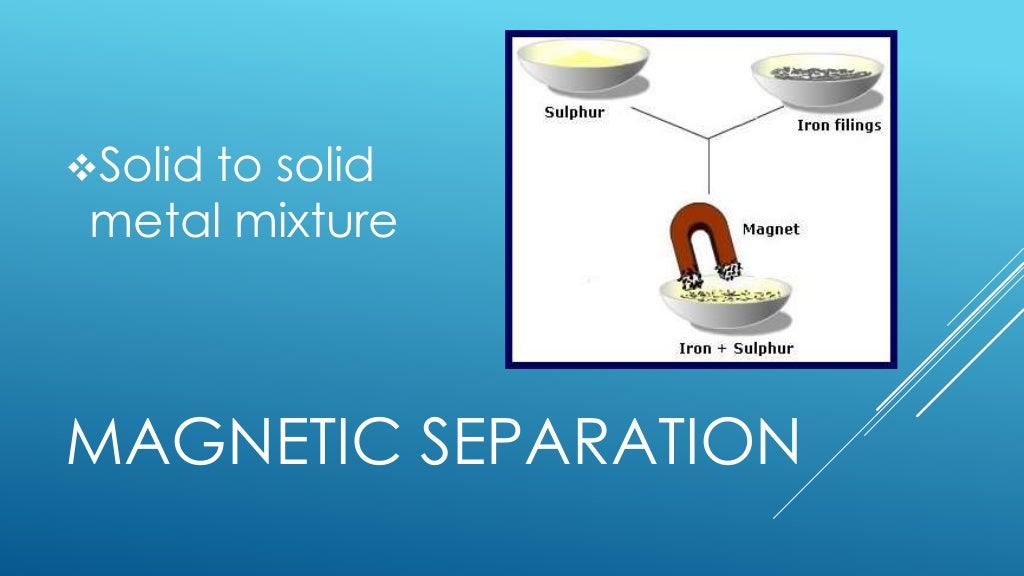
But still, an alloy is considered as a mixture because Alloys: Alloys are homogeneous mixtures of metals and cannot be separated into their components by physical methods.This shows that particles of sugar or salt are evenly distributed in the solution. For example, lemonade tastes the same throughout. In a solution there is homogeneity at the particle level. But, we can also have solid solutions (alloys) and gaseous solutions (air). Usually we think of a solution as a liquid that contains either a solid, liquid or a gas dissolved in it. You come across various types of solutions in your daily life.
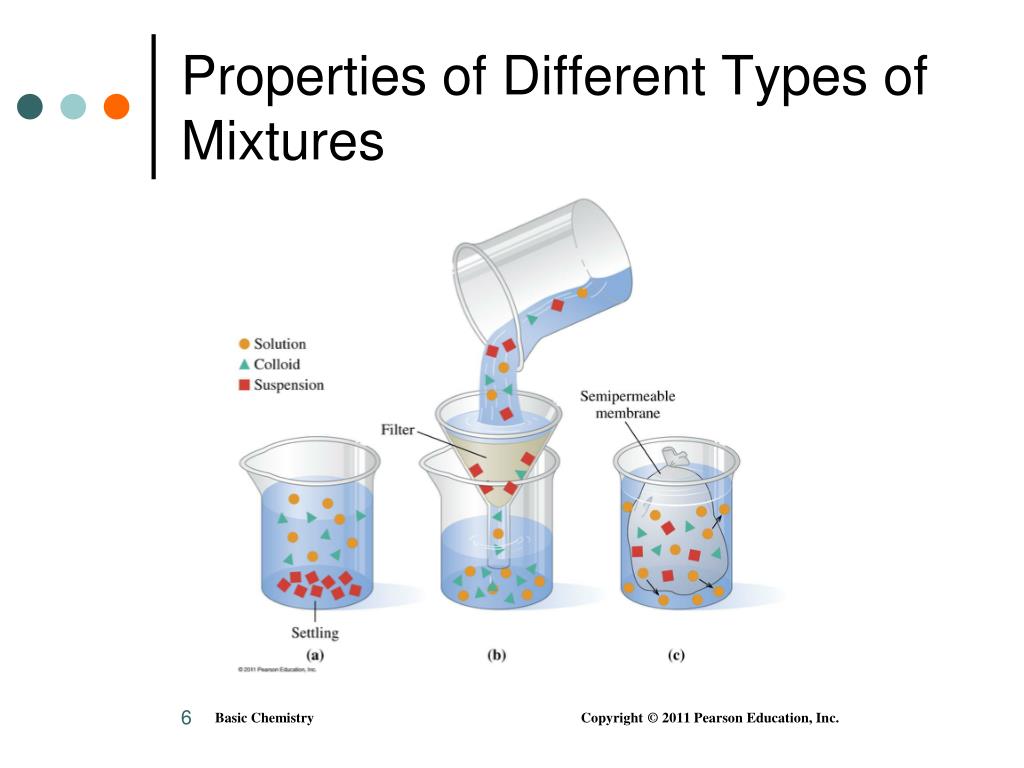
#Which three mixtures will separate when undisturbed full
−One spatula full of copper sulphate to group B. − Few crystals of copper sulphate to group A.

Though both the groups have obtained copper sulphate solution but the intensity of colour of the solutions is different. Compare the colour of the solutions of the two groups. Some other examples of such mixtures are: (i) salt in water and (ii) sugar in water. Such mixtures are called homogeneous mixtures or solutions. Groups A and B have obtained a mixture which has a uniform composition throughout.Report the observations on the uniformity in colour and texture.Groups C and D can take different amounts of copper sulphate and potassium permanganate or common salt (sodium chloride) and mix the given components to form a mixture.Group B takes 50 mL of water and two spatula full of copper sulphate powder in a beaker. Group A takes a beaker containing 50 mL of water and one spatula full of copper sulphate powder.Let us divide the class into groups A, B, C and D.2.1.1 TYPES OF MIXTURESĭepending upon the nature of the components that form a mixture, we can have different types of mixtures. Therefore, we can say that a mixture contains more than one substance. Whatever the source of a substance may be, it will always have the same characteristic properties.

Soft drink and soil are not single substances. Similarly, sugar isĪ substance because it contains only one kind of pure matter and its composition is the same throughout. However, sodium chloride is itself a substance and cannot be separated by physical process into its chemical constituents. We know that dissolved sodium chloride can be separated from water by the physical process of evaporation. A substance cannot be separated into other kinds of matter by any physical Mixtures are constituted by more than one kind of pure form of matter, known as a Two or more pure components, for example, sea water, minerals, soil etc. A pure substance consists of a single type of particles.Īs we look around, we can see that most of the matter around us exist as mixtures of When a scientist says that something is pure, it means that all the constituent particles of that substance are the same in their chemical nature. Milk is actually a mixture of water, fat, proteins etc. Things are actually mixtures of different substances and hence not pure. Have you ever noticed the word ‘pure’ written on the packs of these consumables?įor a common person pure means having no adulteration.


 0 kommentar(er)
0 kommentar(er)
